
What about this carbon right here? It looks like it mightīe a chirality center. All of these carbons have two hydrogens bonded to them, so that's Then we look for chiralityĬenters or chiral centers. Two hydrogens on this carbon all the way around our rings. So there are zero chiralityĬenters in this molecule. That has to be sp3 hybridized, giving you a tetrahedral geometry, and we don't have four different thingsīonded to that carbon, so none of these carbonsĪre chirality centers. We know from earlier videos, that's an sp2 hybridized carbon with trigonal planar geometry. Let's focus in on this carbon right here. The right, three hydrogens, so there's no way it couldīe a chirality center. So that carbon is not a chirality center. For our next example, let me go ahead and draw in the carbons, so we have three carbons. So, one chirality center in this alcohol.
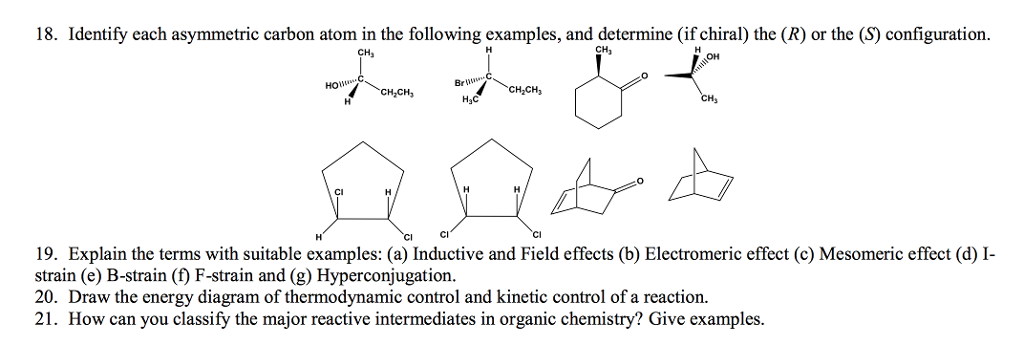
Three of the same thing, so that carbon is not a chirality center. And then finally this carbon over here with three hydrogens,
So I have two hydrogens on this carbon, so that's not a chirality center. I'm going to draw in the other hydrogens. Groups attached to that carbon, so that carbon is a chirality center. There's a methyl group on this side, so that's one different group. We have four different groupsĪttached to this carbon, so I'm going to mark This next carbon here, we have an OH, and then we also have a I need four different groups,Īnd I have three of the same thing on that carbon, So there's no way that'sĪ chirality center. So, let's look for someĬhirality centers in these molecules, and we'll start Sp3 hybridized carbon that has four different "chirality center" here, but you also might hear "chiral center" or "stereo center" or "stereogenic center" or "assymetric center," and they're all pretty much referring to the same concept: a tetrahedral carbon, In summary, the naproxen EF measurements in wastewater effluents show that naproxen is a suitable alternative for evaluating the removal efficiency of treatment systems because its enantioselective degradation is similar under prevailing aerobic and anaerobic conditions.The concept of chirality, the next skill is to be able Hence, under prevailing aerobic conditions, S-ibuprofen degraded faster than R-ibuprofen, whereas under prevailing anaerobic conditions, the degradation was not enantioselective. Injection experiments of ibuprofen in constructed wetlands at microcosm and pilot-scale followed similar trends. The lack of correlation found for ibuprofen was attributable to the fact that its enantioselective degradation kinetics were different under prevailing aerobic and anaerobic conditions. Of the two chiral pharmaceuticals, naproxen was the only one whose effluent EF correlated with its removal efficiency (p<0.05). The enantiomeric fraction (EF=S/(S+R)) in the raw sewage and effluents of various wastewater treatments were found to be compound-dependent (i.e. Ibuprofen and naproxen have an asymmetric carbon atom and, consequently, two enantiomeric forms (i.e.

In addition, injection experiments were carried out with racemic ibuprofen at microcosm- and pilot-scale constructed wetlands. The enantioselective degradation of ibuprofen and naproxen enantiomers was evaluated in five different wastewater treatment systems, including three constructed wetlands (vertical- and horizontal-flow configurations), a sand filter and an activated sludge wastewater treatment plant.


 0 kommentar(er)
0 kommentar(er)
